
The above simple theory of valence is inadequate in at least three ways. Valence electrons are the electrons of an atom that can participate in chemical bonding, for example, for H and He the 1 s electrons, for Li through Ne the 2 s and 2 p electrons, and for Na the 3 s electrons. For discussion of a bond of special importance in biology See also: Hydrogen bond.

Multiple bonds between atoms are common and important examples are the carbon-carbon bond and the carbon-oxygen bonds in ethylene and carbon dioxide. See also: Chemical bonding Electronegativityīonds involving one or three electrons are known, but they are rare H 2 + and HeH are examples. Bond type can be inferred from both chemical and physical evidence. An ionic bond X +Y − will be more stable the less the ionization potential of X and the greater the affinity of Y for electrons, that is, when X is a metallic element from the lower left corner of the periodic table and Y is a nonmetallic element from the upper right corner.
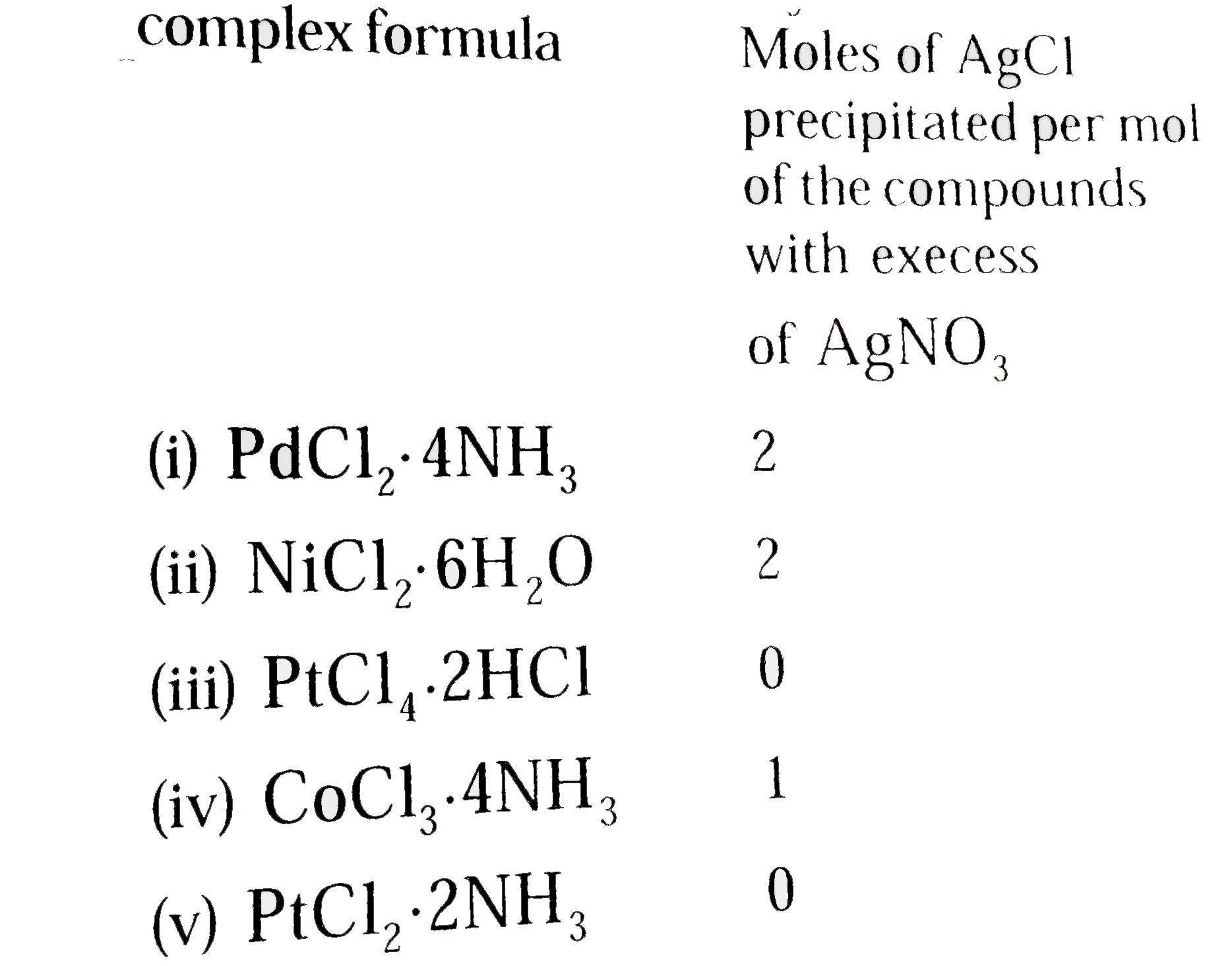
Bonds intermediate in type are possible the bond in hydrogen fluoride (HF) is between covalent and ionic. If there is complete transfer of electrons from one atom to another, the bond is electrovalent or ionic, as in sodium fluoride (NaF). It is coordinate covalent if both electrons come from one atom, as the boron-nitrogen bond in the compound F 3B NH 3. The bond between two atoms is covalent if one electron in the bonding electron pair comes from each atom, as in H:H or the CH bonds in CH 4. The next inert gas is argon (Ar), with a closed shell of 2 + 8 + 8 = 18 electrons, followed by krypton (Kr) with 2 + 8 + 18 + 8 = 36 electrons, and the others. The chemically inert gases helium (He) and neon (Ne) are characterized by closed shells of 2, and 2 + 8 = 10 electrons, respectively. The 3 s state is still higher, in the M shell. Next in energy are 2 s and 2 p, making up the L shell. The lowest energy orbit is 1 s, forming the K shell. To cover just part of the periodic table, occupation of orbits by electrons in the lighter atoms are shown in the table, where the symbol 2 p stands for three distinct orbits of the same energy and shape but differently oriented in space. When the consequences of these ideas are worked out, there actually emerges the periodic classification of the elements. See also: Atomic structure and spectra Electron configuration Furthermore, not more than two electrons can move in one orbit at once. A special quantum effect is operative at the atomic level, however, which possesses no analogy in the motions of planets not all orbits are possible for an electron, but only those for which the angular momentum of the electron as it moves about the nucleus is an integer multiple of h/2π, where h = 6.63 × 10 −34 J is Planck's constant, and for which the energy is similarly quantized.

Bohr, electrons in an atom move in orbits much like the orbits of planets about a sun, held to the nucleus by electrical attractions for it, prevented from falling into it by centrifugal forces. Understanding of molecule formation requires an understanding of the electronic structure of atoms.


 0 kommentar(er)
0 kommentar(er)
